Contraindications
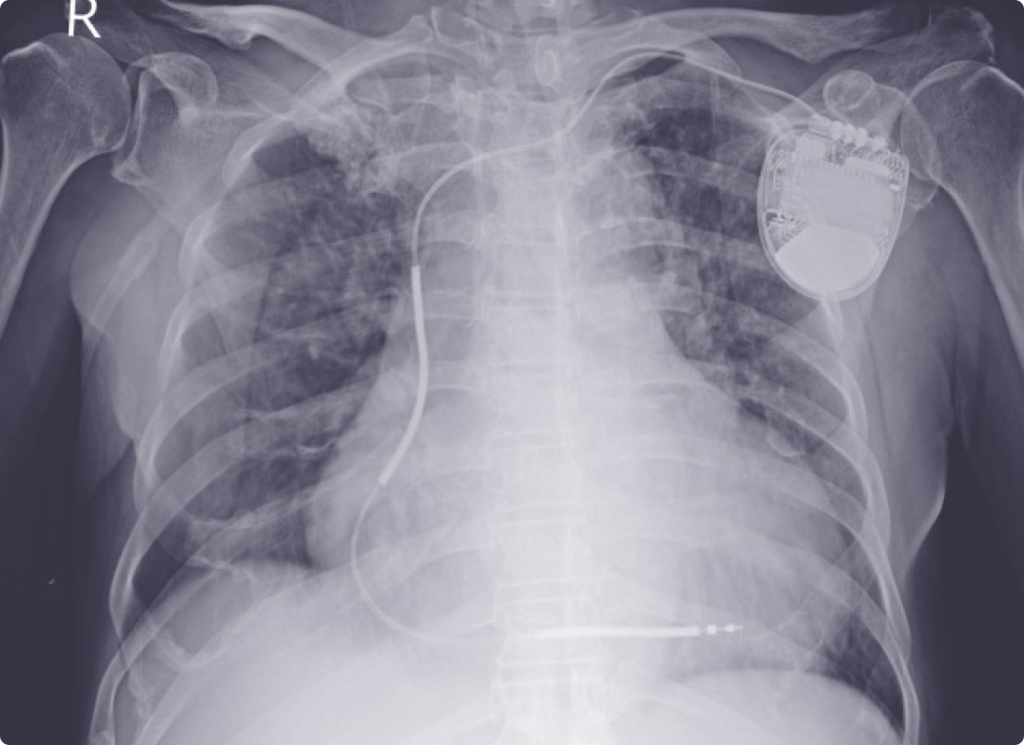
The only absolute contraindication for use of a PEMF device is placing an active applicator over implanted electrical devices like pacemakers, cochlear implants, intrathecal pumps, etc., because the magnetic field can shut the device off or otherwise interfere with its function. Seek caution in situations such as this.
PEMFs are contraindicated in organ transplant patients. This is because these people are on immune suppression medications to prevent organ rejection. We do not want to risk adversely affecting the immune suppression/rejection process. There is a chance that PEMFs may actually stimulate or activate a more aggressive rejection process by stimulating the immune system.

Cautions
Safety of PEMFs has not been established in pregnancy, although there is no evidence of harm. Most manufacturers warn against the use of their device during pregnancy.
PEMFs should be used with caution in Grave’s disease or in the case of active bleeding.
Do not use high intensity PEMFs over breast implants. Given the risk of agitating the plastic or silicone in breast implants resulting in possible thinning and risk of leakage, high-frequency PEMFs beyond 100 Hz is probably also not desirable for treatment duration longer than an hour at a time.
PEMFs should be used with caution in Grave’s disease or in the case of active bleeding.
Do not use high intensity PEMFs over breast implants. Given the risk of agitating the plastic or silicone in breast implants resulting in possible thinning and risk of leakage, high-frequency PEMFs beyond 100 Hz is probably also not desirable for treatment duration longer than an hour at a time.

